MULTI-STAGE INSPECTION OF VALVES
FOR THE MEDICAL SECTOR
In the medical sector, the reliability of components is crucial, especially when they are involved in critical processes such as the cleaning of dialysis devices. The valves used in these systems must exhibit impeccable quality to ensure optimal sterilization and to avoid any risk of contamination. In light of these high demands, a manufacturer turned to an automated inspection solution to ensure total compliance of its products.

THE CHALLENGE
The valves used in dialysis devices are made up of 12 assembled components. During this assembly, it is possible for impurities to infiltrate the valve, and defects to appear.
The client wanted to implement quality control on three different sides of the valve :
- Appearance control : detection of impurities, defects, pollution
- Verification of the presence of multiple components
- Validation of screw fastening compliance
PROJECT COMPLEXITY
CODA Systèmes intervened during the project, after an Italian integrator had already installed the robots, the machines, and the vision programs dedicated to the inspection of an old valve reference. These initial references were white and opaque products.
New references for transparent valves were then developed. It became necessary to reliably detect defects and impurities that could be inside the component, a task much more complex due to its transparency.
This evolution has introduced several major constraints:
- Methodology imposed by the previous integrator: the technical choices and the initial framework could not be modified.
- Integration of a new valve reference : the transparency of the product made defect detection significantly more difficult and required optimization of the vision tools.
- Improvement of existing programs without additional cost to the client : it was necessary to adapt the control programs for this new reference without modifying the machine already installed for the old version, in order to avoid any extra costs.
In addition, there was a critical requirement : some defects were sometimes invisible to the naked eye, and certain impurities could become lodged in the internal structure of the component.
If a single piece was non-compliant, the end customer would return the entire batch, resulting in a direct loss for the company. It was therefore essential to establish a reliable and reproducible inspection process.
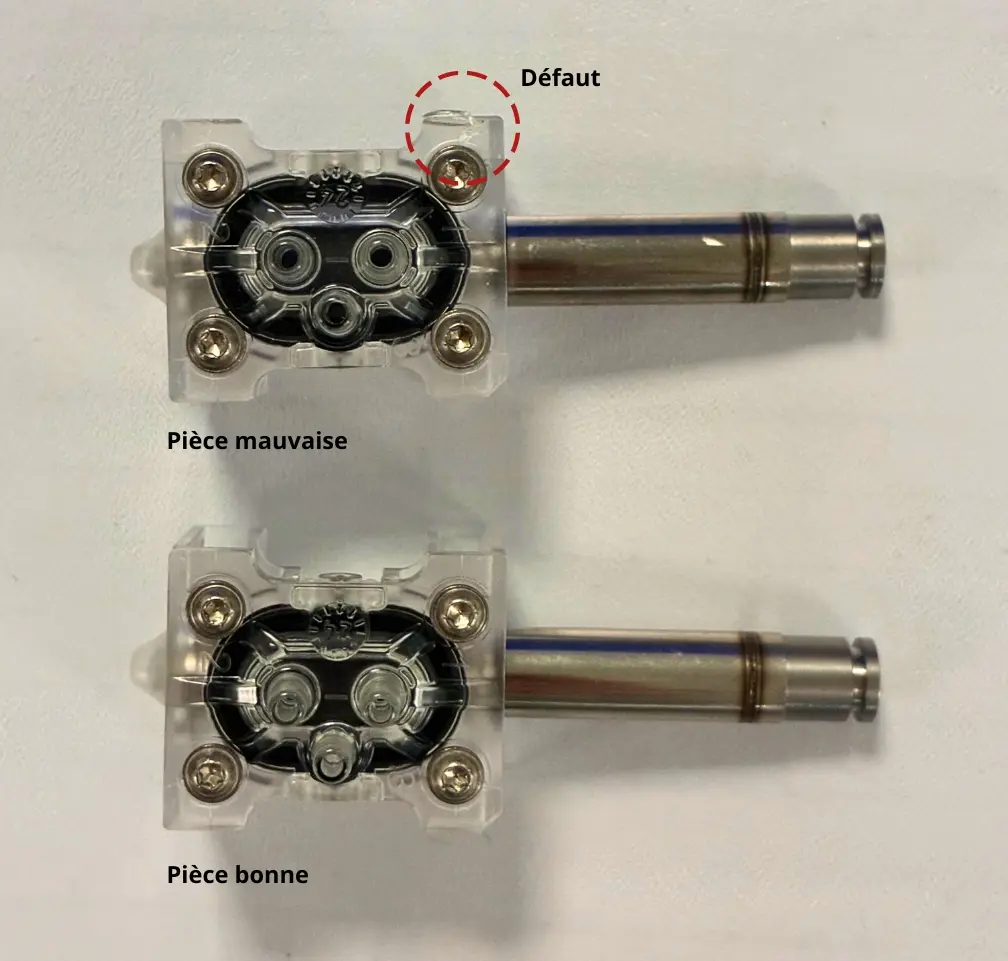
METHODOLOGY
To address these challenges, CODA Systèmes optimized the program initially designed for opaque white valves, and then developed new specific programs for the transparent body of the new reference.
The objective was to ensure complete control of the product on all its surfaces, despite the complexity introduced by the transparency of the material.
It was therefore necessary to implement several controls:
- Appearance and assembly inspection : detection of impurities, stains, contamination, as well as shocks and breakages
- Detection of the presence of certain elements
This methodology was made possible by the implementation of a defect detection tool based on machine learning, (“Autoteach inspection”), allowing each part to be compared to a compliant model.

RESULTS
Almost nonexistent false reject rate.
Stable, repeatable inspection that meets the critical requirements of the medical sector.
Securing the control process and reducing customer return risks.

UNE QUESTION ?
Si vous souhaitez partager un projet ou avoir plus d’informations, notre équipe se tient à votre disposition.
CONTACTER CODA